For the millions with sleep apnea, the traditional treatment option is a CPAP machine. While CPAP is often effective, patients struggle to continue using it. Many patients become non-compliant, and then the symptoms and risks associated with poor sleep return, such as increased risk for heart attack, stroke, weight gain, high blood pressure and heart failure.
For those who have trouble with the masks, hoses, sleeping positions, etc. of positive airway pressure (PAP) therapy, newly FDA-approved treatment options have arrived: implantable breathing stimulators.
One such treatment is called the Inspire® upper airway stimulation (UAS) therapy, meant for treating obstructive sleep apnea. Inspire therapy is the first implantable device for treating OSA. In contrast to CPAP, Inspire therapy works from inside the body and with a patient’s natural breathing process. The implantable system includes a small generator, a sensing lead and a stimulation lead. Turned on by a handheld remote, it delivers mild stimulation to key airway muscles, which keeps the airway open during sleep.
The Inspire system is meant for patients with moderate to severe obstructive sleep apnea (OSA) who are unable to use continuous positive airway pressure (CPAP) therapy.
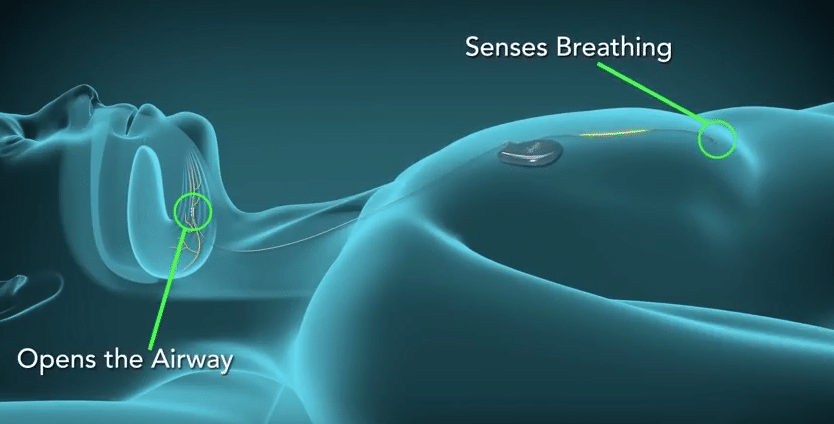
Another implantable device is the Remedē® System for treating central sleep apnea. The Remedē System stimulates a nerve located in the chest that is responsible for sending signals to the diaphragm to stimulate breathing. The system is comprised of a battery pack surgically placed under the skin in the upper chest area and small, thin wires that are inserted into the blood vessels in the chest near the nerve (phrenic) that stimulates breathing. The system monitors the patient’s respiratory signals during sleep and stimulates the nerve to move the diaphragm and restore normal breathing. It is placed during a minimally invasive outpatient procedure by a cardiologist.
Respicardia-The remedē® System- 2018 from Respicardia® on Vimeo.
Does It Work?
The FDA evaluated data from 141 patients to assess the effectiveness of the Remedē System in reducing apnea hypopnea index (AHI), a measure of the frequency and severity of apnea episodes. In their press release, the FDA stated that “after six months, AHI was reduced by 50 percent or more in 51 percent of patients with an active Remedē System implanted. AHI was reduced by 11 percent in patients without an active Remedē System implanted.”
Additional studies showed that 87% of patients with this system had a reduction in the number of sleep apnea events.
Important to note is that the Remedē System is not intended for use in patients with obstructive sleep apnea, as the Inspire system is.
Studies conducted on Upper Airway Stimulation, as used in the Inspire therapy, also show positive results. The STAR Trial published in the New England Journal of Medicine found a 78% reduction in sleep apnea events per hour in patients undergoing UAS.
Other Studies:
https://academic.oup.com/sleep/article/38/10/1593/2468601
http://erj.ersjournals.com/content/early/2014/09/03/09031936.00059414.short